Definition of a fluid
Definition of a Fluids
A fluid is defined as a substance that deforms continuously whilst acted upon by any force tangential to the area on which it acts. Such a force is termed a shear force, and the ratio of the shear force to the area on which it acts is known as the shear stress (see fig. 1). The characteristic that distinguishes a fluid from a solid is its inability to resist deformation under an applied shear stress (a tangential force per unit area). When a fluid is at rest neither shear forces nor shear stresses exist in it. A solid, on the other hand, can resist a shear force while at rest. In a solid, the shear force may cause some initial displacement of one layer over another, but the material does not continue to move indefinitely and a position of stable equilibrium is reached.
Definition 1
Fluid is any substance that deforms continuously when subjected to a shear stress, no matter how small. Shear forces are possible only while relative movement between layers is taking place.
Fluids may be sub-divided into liquids and gases. A fixed amount of a liquid has a definite volume which varies only slightly with temperature and pressure. If the capacity of the containing vessel is greater than this definite volume, the liquid occupies only part of the container, and it forms an interface separating it from its own vapor, the atmosphere
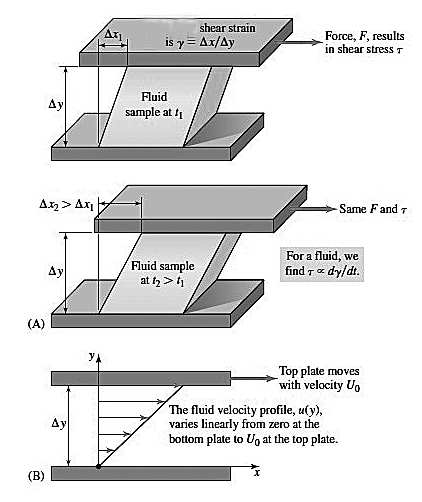
Fig. 1
Figure 1: (A) The displacement and the corresponding shear strain increase linearly with time. For a fluid, the relationship between shear stress and shear strain is proportional. (B) The fluid velocity in the x direction, u, is a function of the y coordinate. The velocity u(y) varies linearly from 0 at the bottom plate to U0 at the top plate .
Gas- a fixed amount of a gas, by itself in a closed container, will always expand until its volume equals that of the container. Only then can it be in equilibrium.
In the analysis of the behavior of fluids an important difference between liquids and gases is that, whereas under ordinary conditions liquids are so difficult to compress that they may for most purposes be regarded as incompressible, gases may be compressed much more readily. Where conditions are such that an amount of gas undergoes a negligible change of volume, its behavior is similar to that of a liquid and it may then be regarded as incompressible. If, however, the change in volume is not negligible, the compressibility of the gas must be taken into account in examining its behavior.
Liquids have much greater densities than gases. As a consequence, when considering forces and pressures that occur in fluid mechanics, the weight of a liquid has an important role to play. Conversely, effects due to weight can usually be ignored when gases are considered.
The different characteristics of solids, liquids and gases result from differences in their molecular structure. All substances consist of vast numbers of molecules separated by empty space. The molecules have an attraction for one another, but when the distance between them becomes very small (of the order of the diameter of a molecule) there is a force of repulsion between them which prevents them all gathering together as a solid lump.
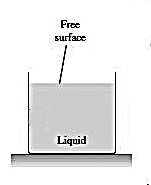
Fig. 2
Figure 2: The liquid occupies only part of the container, and it forms an interface separating it from its own vapor.
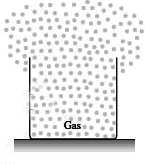
Fig. 3
Figure 3: A fixed amount of a gas, by itself in a closed container, will always expand until its volume equals that of the container.

