Engineering Materials Introduction
Engineering Materials Introduction Selection:
Let’s now start by looking at the basic requirements for selecting materials that are suitable for a particular application. For example figure 1 shows a connecter joining electric cables. The plastic casing has been partly cut away to show the metal connector. Plastic is used for the outer casing because it is a good electrical insulator and prevents electric shock if a person touches it. It also prevents the conductors touching each other and causing a short circuit. As well as being a good insulator the plastic is cheap, tough, and easily moulded to shape. It has been selected for the casing because of these properties – that is, the properties of toughness, good electrical insulation, and ease of moulding to shape. It is also a relatively low cost material that is readily available.
The metal joining piece and its clamping screws are made from brass. This metal has been chosen because of its special properties. These properties are good electrical conductivity, ease of extruding to shape, ease of machining (cutting to length, drilling and tapping the screw threads), adequate strength and corrosion resistance. The precious metal silver is an even better conductor, but it would be far too expensive for this application and it would also be too weak and soft.
Figure 1. The electrical connector.
Another example as in figure 2 shows the connecting rod of a motor car engine. This is made from a special steel alloy. This alloy has been chosen because it combines the properties of strength and toughness with the ability to be readily forged to shape and finished by machining.

Figure 2. The connecting rod of motor car engine.
Thus the reasons for selecting the materials in the above examples can be summarized as:
- Commercial factors such as: Cost, availability, ease of manufacture.
- Engineering properties of materials such as: Electrical conductivity, strength, toughness, ease of forming by extrusion, forging and casting, machinability and corrosion resistance.
Engineering Materials:
Almost every substance known to man has found its way into the engineering workshop at some time or other. The most convenient way to study the properties and uses of engineering materials is to classify them into ‘families’ as shown in figure below:
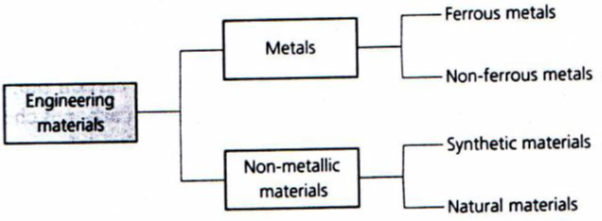
Figure 3. Classification of engineering materials.
Metals
Ferrous metals
- These are metals and alloys containing a high proportion of the element iron.
- They are the strongest materials available and are used for applications where high strength is required at relatively low cost and where weight is not of primary importance.
- As an example of ferrous metals such as: bridge building, the structure of large buildings, railway lines, locomotives and rolling stock and the bodies and highly stressed engine parts of road vehicles.
- The ferrous metals themselves can also be classified into “families’, and these are shown in figure 4.
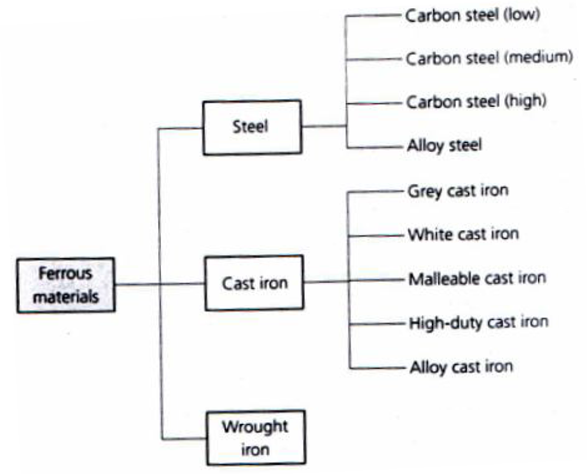
Figure 4. Classification of ferrous metals.
Non – ferrous metals
- These materials refer to the remaining metals known to mankind.
- The pure metals are rarely used as structural materials as they lack mechanical strength.
- They are used where their special properties such as corrosion resistance, electrical conductivity and thermal conductivity are required. Copper and aluminum are used as electrical conductors and, together with sheet zinc and sheet lead, are use as roofing materials.
- They are mainly used with other metals to improve their strength.
- Some widely used non-ferrous metals and alloys are classified as shown in figure 5.
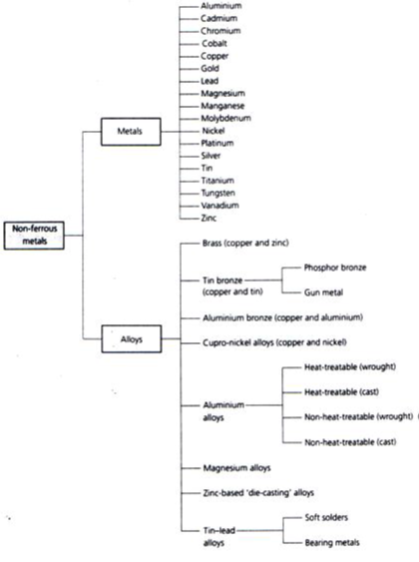
Figure 5. Classification of non-ferrous metals and alloys.
Non – metallic (synthetic materials )
- These are non – metallic materials that do not exist in nature, although they are manufactured from natural substances such as oil, coal and clay. Some typical examples are classified as shown in figure 6.

Figure 6. classification of synthetic materials.
- They combine good corrosion resistance with ease of manufacture by moulding to shape and relatively low cost.
- Synthetic adhesives are also being used for the joining of metallic components even in highly stressed applications.
Non – metallic (Natural materials )
Such materials are so diverse that only a few can be listed here to give a basic introduction to some typical applications.
- Wood: This is naturally occurring fibrous composite material used for the manufacture of casting patterns.
- Rubber :This is used for hydraulic and compressed air hoses and oil seals. Naturally occurring latex is too soft for most engineering uses but it is used widely for vehicle tyres when it is compounded with carbon black.
- Glass : This is a hardwearing, abrasion-resistant material with excellent weathering properties. It is used for electrical insulators, laboratory equipment, optical components in measuring instruments etaand, in the form of fibers, is used to reinforce plastics. It is made by melting together the naturally occurring materials : silica (sand), limestone (calcium carbonate ) and soda (sodium carbonate).
- Emery : This is a widely used abrasive and is a naturally occurring aluminum oxide. Nowadays it is produced synthetically to maintain uniform quality and performance.
- Ceramic: These are produced by baking naturally occurring clays at high temperatures after moulding to shape. They are used for high – voltage insulators and high – temperature – resistant cutting tool tips.
- Diamonds: These can be used for cutting tools for operation at high speeds for metal finishing where surface finish is greater importance. For example, internal combustion engine pistons and bearings. They are also used for dressing grinding wheels.
- Oils : Used as bearing lubricants, cutting fluids and fuels.
- Silicon : This is used as an alloying element and also for the manufacture of semiconductor devices.
These and other natural, non-metallic materials can be classified as shown in figure 7.
Composite Materials
These are materials made up from, or composed of, a combination of different materials to take overall advantage of their different properties. In man-made composites, the advantages of deliberately combining materials in order to obtain improved or modified properties was understood by ancient civilizations. An example of this was the reinforcement of air-dried bricks by mixing the clay with straw. This helped to reduce cracking caused by shrinkage stresses as the clay dried out. In more recent times, horse hair was used to reinforce the plaster used on the walls and ceiling of buildings. Again this was to reduce the onset of drying cracks.
Nowadays, especially with the growth of the plastics industry and the development of high-strength fibers, a vast range combinations of materials is available for use in composites.
For example, carbon fiber reinforced frames for tennis rackets and shafts for golf clubs have revolutionized these sports.
Figure 7. Classification of natural materials.
Factors Affecting Materials Properties:
The following are the more important factors which can be influence the properties and performance of engineering materials.
Heat treatment
This is the controlled heating and cooling of metals to change their properties to improve their performance or to facilitate processing.
An example of heat treatment is the hardening of a piece of highcarbon steel rod. If it is heated to dull red heat and plunged into cold water to cool it rapidly (quenching), it will become hard and brittle. If it is again heated to dull red heat but allowed to cold very slowly it will become softer and less brittle (more tough). In this condition it is said to be annealed.
After the heat treatment happened on the material it will be in its best condition for flow forming, during flow forming (working) the grains will be distorted and this will result in most metals becoming work hardened if flow formed at room temperature. To remove any locked in stresses resulting from the forming operations and to prepare the material for machining, the material has to be normalized.
Processing
Hot –and cold working process will be referred to understand what is meant by terms hot and cold working as applied to metals. Figure 8 shows examples of hot and cold working.

Figure 8. Examples of (a) hot-working and (b) cold-working process.
Metal is hot worked or cold worked depending upon the temperature at which it is flow formed to shape. These temperatures are not easy to define, for instance, lead hot works at room temperature and can be beaten into complex shapes without cracking , but steel does not hot work until it is red hot.
When metal are examined under the microscope it can be seen that they consist of very small grains. When most metals are bent or worked at room temperature (cold worked) these grains become distorted and the metal becomes hard and brittle.
When metals are hot worked the crystals are also distorted, however, they reform instantly into normal crystals because the process temperature is above the temperature of re-crystallisation for the metal being used and work hardening does not occur. This cold working is the flow forming of metals below the temperature the re-crystallisation, whilst hot working is the flow forming of metals above the temperature of recrystallisation.
Environmental Reactions
The properties of materials can also be effected by reaction with environment in which they are used. For example:
Resting of steel
Unless steel structures are regularly maintained by rest neutralization and painting process, resting will occur. The rest will eat into the steel, reduce its thickness and, therefore, its strength. In extreme cases an entire structure made from steel may be eaten away.
Dezincification of brass
Brass is an alloy of copper and zinc and when brass is exposed to a marine environment for along time, the salt in the sea water pray react with the zinc content of the brass so as remove it and leave it behind on spongy, porous mass of copper. This obviously weakness the material which fails under normal working conditions.
Degradation of plastic
Many plastic degrade and become weak and brittle when exposed to the ultraviolet content of sunlight. Special dyestuffs have to be incorporated into the plastic to filter out these harmful rays.

