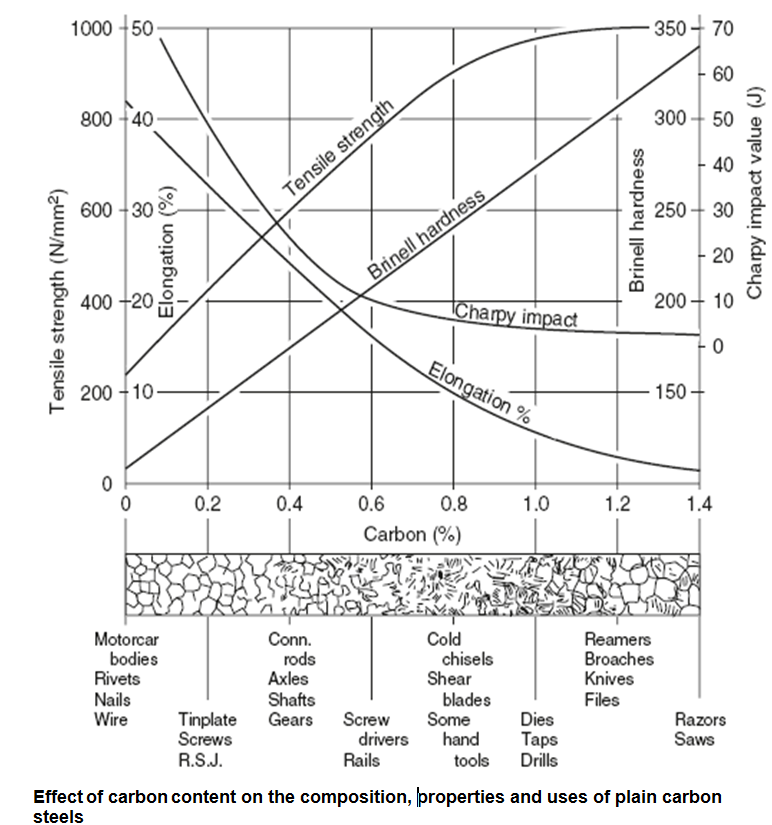
Ferrous metals – plain carbon steels
Ferrous metals are based on the metallic element iron (Latin ferrum =iron). The iron is associated with carbon, either as a solid solution or as the chemical compound iron carbide (cementite). In the case of cast irons, some amount of carbon may be uncombined (free) in the form of flake graphite. In addition to carbon, other elements may also be present. These may be impurities such as sulphur and phosphorus which weaken the metal and are kept to a minimum. Alloying elements are added to enhance the performance of the metal (e.g. chromium and nickel).
Plain carbon steels consist mainly of iron and carbon, and are the simplest of the ferrous metals. Some manganese will also be present to neutralize the deleterious effects of the sulphur and to enhance the grain structure. It is not present in sufficient quantity to be considered as an alloying element.
The amount of carbon present affects the properties of the steel as shown below. The maximum amount of carbon which can remain combined with the iron at all temperatures is 1.7%. In practice an upper limit of 1.2–1.4% is set to ensure a margin of safety. A steel, by definition, must contain no free carbon.
Low-carbon steels
These have a carbon content 0.1–0.3% plus impurities, plus some manganese to neutralize the effect of any sulphur content left over from the extraction process. Such steels cannot be directly hardened by heat treatment, but they can be readily carburized and case hardened. The lower-carbon steels in this category are used for steel sheets for pressing out such components as motorcar body panels as they have a high ductility. The lower-carbon steels in this category are also made into drawn wire rod and tube. The higher-carbon steels in this category are stiffer and less ductile, and are used for general workshop bars, plates and girders. Low-carbon steels are substantially stronger than wrought iron which is no longer considered to be a structural material.
Medium-carbon steels
(a) Carbon content 0.3–0.5%: Such steels can be toughened by heat treatment (heating to red heat and quenching – rapid cooling – in water). They are used for crankshaft and axle forgings where cost is important and the service requirements do not warrant stronger but more expensive alloy steels.
(b) Carbon content 0.5–0.8%: These are used for vehicle leaf springs and garden tools. Such steels can be quench hardened by heat treatment as above.
High-carbon steels
All high carbon steels can be hardened to a high degree of hardness by heating to a dull red heat and quenching. The hardness and application depend on the carbon content, the rate of cooling from the hardening temperature and the degree of tempering after hardening:
(a) Carbon content 0.8–1.0%; used for coil springs and wood chisels.
(b) Carbon content 1.0–1.2%; used for files, drills, taps and dies.
(c) Carbon content 1.2–1.4%; used for fine-edge tools (knives, etc.).