Fluid as a continuum
The most fundamental idea we will need is the continuum hypothesis. In simple terms this says that when dealing with fluids we can ignore the fact that they actually consist of billions of individual molecules (or atoms) in a rather small region, and instead treat the properties of that region as if it were a continuum. By appealing to this assumption we may treat any fluid property as varying continuously from one point to the next within the fluid; this clearly would not be possible without this hypothesis. To understand how physical quantities are defined in the continuum model we consider the following experiment to observe hob density of fluid is related to its molecular structure. At time t we consider a cube with the width α occupied by fluid centered at x0 .The average density of the fluid is ρα=Mα/α3 , where Mα is the mass of the fluid inside of the cube. To define the density ρ(x0 , t) it is examined what happens as α approaches zero. The graph fig. 4) shows the results. In region II, since there are many particles inside the cube, the average density ρα vary very little. If, however α were on the order of molecular distances, ∼ 10e−9 meters, the may be only a few molecules in the cube and one will observe large fluctuations in ρα. Such rapid fluctuations are depicted in region I, It seems unreasonable therefore to define ρ(x0 , t) as the limiting value of ρα as α → 0. Rather ρ(x0 , t) should be defined as
![]()
where α∗ is the value of α where density begin to vary violently, here for example α∗ = 10e−9 meters. In a similar fashion other physical quantities can be considered as point functions in continuously distributed matter without regard to its molecular or atomic structure.
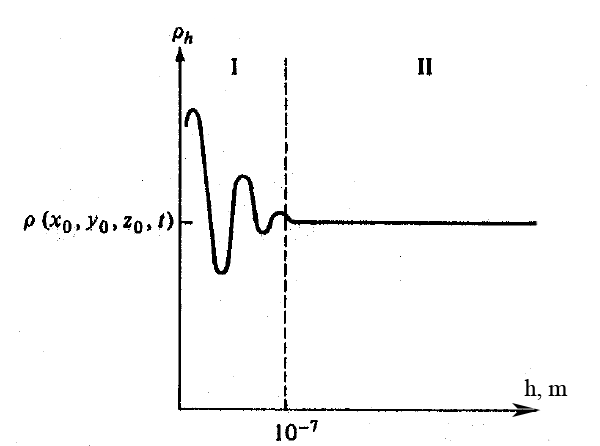
Fig. 4: Graph of the average density ρα versus the with α.

