Mass, force and weight
Mass per unit volume
Mass
- Mass is the quantity of matter in a body. It is the sum total of the masses of all the subatomic particles in that body.
- Matter occupies space and can be solid, liquid or gaseous.
- Unless matter is added to or removed from a body, the mass of that body never varies.
- It is constant under all conditions. There are as many atoms in a kilogram of, say, metal on the moon as there are in the same kilogram of metal on planet Earth. However, that kilogram of metal will weigh less on the moon than on planet Earth (see Section 2.2.4).
- The basic unit of mass is the kilogram (kg). The most commonly used multiple of this basic unit is the tonne (1000 kg) and the most commonly used sub-multiple is the gram (0.001 kg).
Force
To understand how mass and weight are related it is necessary to consider the concept of force. A force or a system of forces cannot be seen; only the effect of a force or a system of forces can be seen. That is:
- A force can change or try to change the shape of an object.
- A force can move or try to move a body that is at rest. If the magnitude of the force is sufficiently great it will cause the body to move in the direction of the application of the force. If the force is of insufficient magnitude to overcome the resistance to movement it will still try to move the body, albeit unsuccessfully.
- A force can change or try to change the motion of a body that is already moving. For example:
-
- The force of a headwind can decrease the speed of an aircraft over the ground, whereas the force of a tailwind can increase the speed of an aircraft over the ground.
- The force of a crosswind can cause a car to swerve off course.
- The effect of any force depends on the following:
-
- The magnitude (size) of the force measured in newtons (N).
- The direction of the force.
- The point of application of the force.
- The ability of a body to resist the effects of the force.
- As already stated a force cannot be seen; however, it can be represented by a vector
Vectors
Quantities can be divided into two categories:
- Scalar quantities
- Vector quantities.
Scalar quantities have magnitude (size) only. For example:
- The speed of a car can be stated as 50 kilometres per hour (km/h).
- The power of an engine can be rated as 150 kilowatts (kW).
- The current flowing in an electric circuit can be 12 amperes (A).
Vector quantities have both magnitude and direction. For example, the velocity of a car is 50 km/h due north from London. Velocity is a vector quantity since it has both magnitude (speed) and direction (due north).
Similarly, vectors can be used to represent forces and systems of forces. Figure 2.1 shows how a vector can be used to represent a force (F) acting on the point (P) in a direction at 30° to the horizontal with a magnitude of 150 newtons (N). Vector diagrams will be considered in detail in Section 2.3.
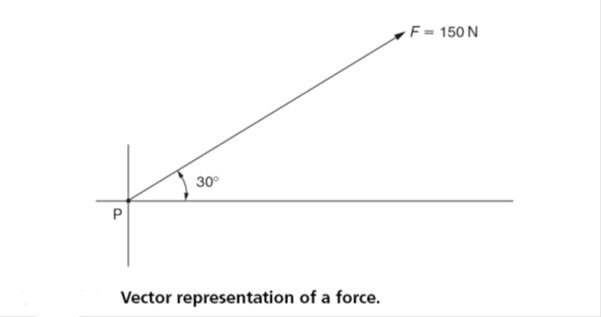
To represent a force by a vector the following information is required:
- The point of application of the force.
- The magnitude (size) of the force.
- The line of action of the force.
- The direction in which the force is acting. The direction in which the force is acting is shown by the arrowhead. This can be at the end of the vector or at the midpoint of the vector, whichever is the more convenient.
Weight
A solid, liquid or gas of mass 1 kg will weigh 9.81 N due to the gravitational attraction of planet Earth. Since planet Earth is not a perfect sphere a mass of 1 kg will weigh slightly more at the poles and slightly less at the equator. However, for all practical purposes, the average weight of a mass of 1 kg is taken to be 9.81 N on planet Earth:
- The force of gravity attracts the mass of any body (solid, liquid or gas) towards the centre of planet Earth.
- The weight of a body is the force of gravity acting on that body.
- Therefore weight is a force measured in newtons.
- The factor 9.81 is the free-fall acceleration of a body due to the force gravity, it is 9.81 metres per second squared (m/s2). Acceleration due to gravity is discussed further in Section 3.6.7.
- Since the weight of a body of mass 1 kg is approximately 9.81 N (average value) on planet Earth, the weight of that body will change on a planet having a different force of gravity or when travelling through space. For example, the gravitational attraction of the Moon is approximately 1/6 that of planet Earth. Therefore a mass of 1 kg will weigh 1/6×9.81=1.64 N on the Moon.
Mass per unit volume (density)
A solid object has a volume of 60 cm3. If the mass of this solid is 600 grams (g) then, by proportion,
1 cm3 has a mass of 600 g/60 cm3=10 gram per cm3 (10 g/cm3)
Since 1 cm3 is unit volume in this instance, then the mass per unit volume of the solid shown is 10 g/cm3. Mass per unit volume of any body is also called the density of that body. The symbol for density is the Greek letter ‘rho’ (ρ). The general expression for mass per unit volume (density) is:
ρ=m/V
where:
ρ=density
m=mass
V=volume
The densities of some common solids, liquids and gases used in engineering are given in Table below.
Weight per unit volume
The weight per unit volume of a substance can be determined by:
(a) direct measurement,
(b) conversion calculation from the density of the substance.
(a) Weight per unit volume by direct measurement:
Weight per unit volume (N/m3)=weight (N)/volume (m3)=W/V
| Density of common substances | ||
| Substance |
Density |
|
| kg/m3 | g/cm3 | |
| Aluminium | 2720 | 2.72 |
| Brass | 8480 | 8.48 |
| Copper | 8790 | 8.79 |
| Cast iron | 7200 | 7.20 |
| Lead | 11350 | 11.35 |
| Nylon | 1120 | 1.12 |
| PVC | 1360 | 1.36 |
| Rubber | 960 | 0.96 |
| Steel | 7820 | 7.82 |
| Tin | 7280 | 7.28 |
| Zinc | 7120 | 7.12 |
| Alcohol | 800 | 0.80 |
| Mercury | 13590 | 13.59 |
| Paraffin | 800 | 0.80 |
| Petrol | 720 | 0.72 |
| Water (pure at 4°C) | 1000 | 1.00 |
| Acetylene | 1.17 | 0.0017 |
| Air (dry) | 1.30 | 0.0013 |
| Carbon dioxide | 1.98 | 0.00198 |
| Hydrogen | 0.09 | 0.00009 |
| Nitrogen | 1.25 | 0.00125 |
| Oxygen | 1.43 | 0.00143 |
(b) Weight per unit volume by conversion calculation:
Weight per unit volume =W/V 1)
Density (ρ)=m/V 2)
Weight=m × 9.81 3)
Substituting equation (3) in equation (2):
Weight per unit volume=(m × 9.81)/V
But:
m/V= ρ
Therefore:
Weight per unit volume= ρ ×9.81
Note:
- This conversion only applies to planet Earth. Anywhere else in space the local value of gravitational acceleration ‘g’ must be used.
- The value for density must be expressed in kilograms per unit volume.
Relative density
The relative density of a substance may be defined as the ratio:
(Density of the substance)/(density of pure water at 4°C )
or
(Mass of the substance)/(mass of an equal volume of pure water at 4°C)
Since relative density is a ratio there are no units.
The relative densities of some common substances used in engineering are given in Table below
| Relative densities of some common substances | |
| Substance | Relative density (d) |
| Aluminium | 2.72 |
| Brass | 8.48 |
| Cadmium | 8.57 |
| Chromium | 7.03 |
| Copper | 8.79 |
| Cast iron | 7.20 |
| Lead | 11.35 |
| Nickel | 8.73 |
| Nylon | 1.12 |
| PVC | 1.36 |
| Rubber | 0.96 |
| Steel | 7.82 |
| Tin | 7.28 |
| Zinc | 7.12 |
| Alcohol | 0.80 |
| Mercury | 13.95 |
| Paraffin | 0.80 |
| Petrol | 0.72 |
| Water (pure @4°C) | 1.00 |
| Water (sea) | 1.02 |
| Acetylene | 0.0017 |
| Air (dry) | 0.0013 |
| Carbon dioxide | 0.00198 |
| Carbon monoxide | 0.00126 |
| Hydrogen | 0.00009 |
| Nitrogen | 0.00125 |
| Oxygen | 0.00143 |
Pressure (fluids)
The pressure acting on the surface area of a body is defined as the normal force per unit area acting on the surface. Normal force means the force acting at right angles to the surface. The SI unit of fluid pressure is the pascal (Pa), where 1 Pa is equal to 1Nm2.
Pressure (p) =F/A
where:
p =pressure in pascals
F =force in newtons acting at right angles (normal) to the surface
A =area of the surface in metres squared
Similarly:
1 MPa =1 MN/m2 =1 N/mm2
Since the pascal is a very small unit a more practical unit is the bar, where:
1 bar =105 pascals.
Note:
- Atmospheric pressure is usually expressed in millibars.
- Gauge pressure is the fluid pressure indicated by a bourdon tube pressure gauge, a U-tube manometer or similar device so that the indicated pressure is additional to the atmospheric pressure.
- Absolute pressure used in physical science and thermodynamics is the pressure relative to a perfect vacuum, that is:
Absolute pressure =atmospheric pressure =gauge pressure

