Measurement
Physics can also be defined as the branch of science dealing with the study of properties of materials. To understand the properties of materials, measurement of physical quantities such as length, mass, time etc., are involved. The uniqueness of physics lies in the measurement of these physical quantities.
Fundamental quantities and derived quantities
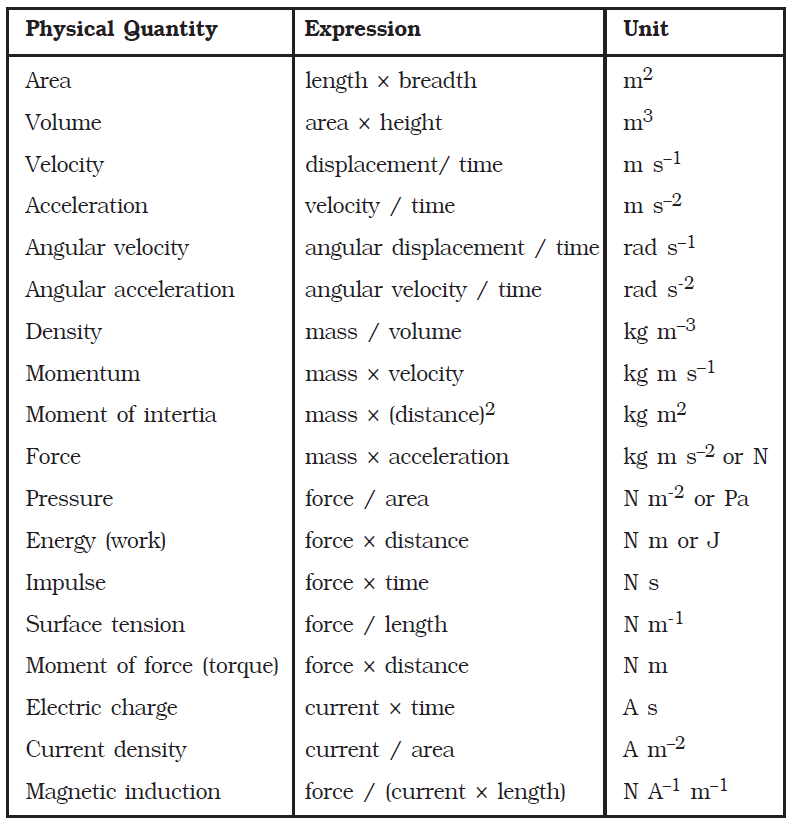
Physical quantities can be classified into two namely, fundamental quantities and derived quantities. Fundamental quantities are quantities which cannot be expressed in terms of any other physical quantity. For example, quantities like length, mass, time, temperature are fundamental quantities. Quantities that can be expressed in terms of fundamental quantities are called derived quantities. Area, volume, density etc. are examples for derived quantities.
Unit
To measure a quantity, we always compare it with some reference standard. To say that a rope is 10 metres long is to say that it is 10 times as long as an object whose length is defined as 1 metre. Such a standard is called a unit of the quantity.
Therefore, unit of a physical quantity is defined as the established standard used for comparison of the given physical quantity. The units in which the fundamental quantities are measured are called fundamental units and the units used to measure derived quantities
are called derived units.
System International de Units (SI system of units)
In earlier days, many system of units were followed to measure physical quantities. The British system of foot−pound−second or fps system, the Gaussian system of centimetre − gram − second or cgs system, the metre−kilogram − second or the mks system were the three systems commonly followed. To bring uniformity, the General Conference on Weights and Measures in the year 1960, accepted the SI system of units. This system is essentially a modification over mks system and is, therefore rationalised mksA (metre kilogram second ampere) system.
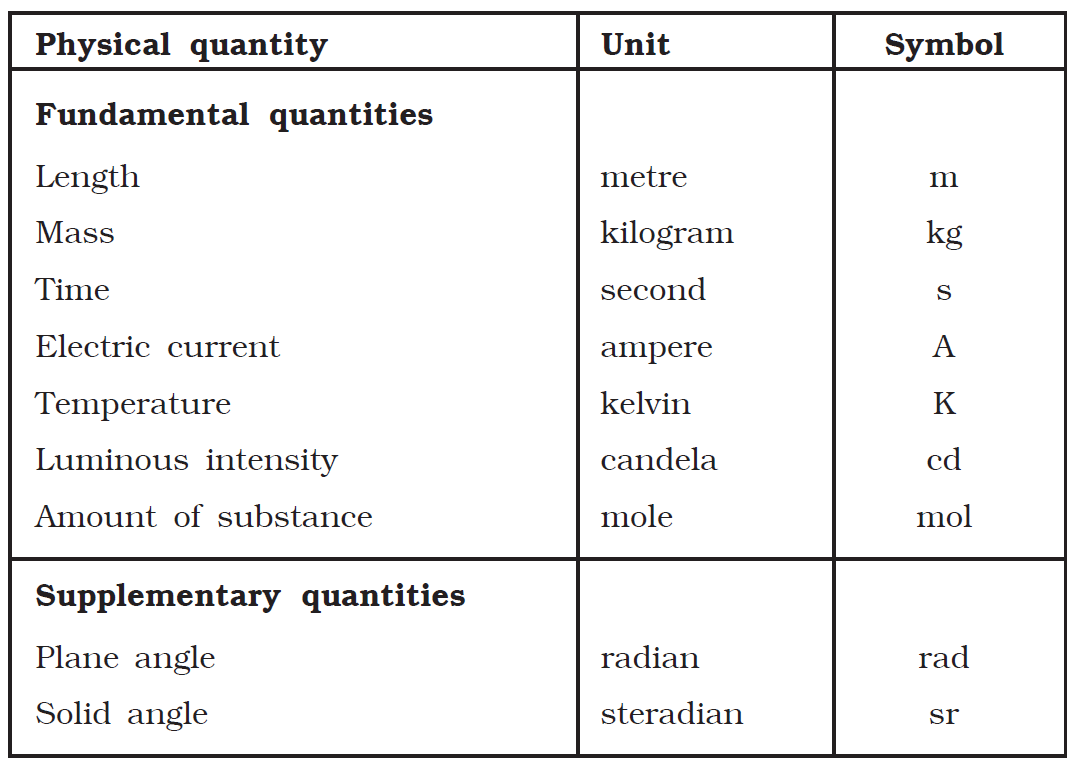
This rationalisation was essential to obtain the units of all the physical quantities in physics. In the SI system of units there are seven fundamental quantities and two supplementary quantities.
SI system of units
Derived quantities and their units
SI standards
Length is defined as the distance between two points. The SI unit of length is metre. One standard metre is equal to 1 650 763.73 wavelengths of the orange − red light emitted by the individual atoms of krypton − 86 in a krypton discharge lamp.
Mass is the quantity of matter contained in a body. It is independent of temperature and pressure. It does not vary from place to place. The SI unit of mass is kilogram. The kilogram is equal to the mass of the international prototype of the kilogram (a plantinum − iridium alloy cylinder) kept at the International Bureau of Weights and Measures at Sevres, near Paris, France.
An atomic standard of mass has not yet been adopted because it is not yet possible to measure masses on an atomic scale with as much precision as on a macroscopic scale.
Time Until 1960 the standard of time was based on the mean solar day, the time interval between successive passages of the sun at its highest point across the meridian. It is averaged over an year. In 1967, an atomic standard was adopted for second, the SI unit of time.
One standard second is defined as the time taken for 9 192 631 770 periods of the radiation corresponding to unperturbed transition between hyperfine levels of the ground state of cesium − 133 atom. Atomic clocks are based on this. In atomic clocks, an error of one second occurs only in 5000 years.
Ampere – The ampere is the constant current which, flowing through two straight parallel infinitely long conductors of negligible cross-section, and placed in vacuum 1 m apart, would produce between the conductors a force of 2 × 10 -7 newton per unit length of the conductors.
Kelvin – The Kelvin is the fraction of 1/273.16 of the thermodynamic temperature of the triple point of water.
Candela – The candela is the luminous intensity in a given direction due to a source, which emits monochromatic radiation of frequency 540 × 10^12 Hz and of which the radiant intensity in that direction is 1/683 watt per steradian.
Mole – The mole is the amount of substance which contains as many elementary entities as there are atoms in 0.012 kg of carbon-12.
Rules and conventions for writing SI units and their symbols
- The units named after scientists are not written with a capital initial letter. For example : newton, henry, watt.
- The symbols of the units named after scientist should be written by a capital letter. For example : N for newton, H for henry, W for watt.
- Small letters are used as symbols for units not derived from a proper name. For example : m for metre, kg for kilogram.
- No full stop or other punctuation marks should be used within or at the end of symbols. For example : 50 m and not as 50 m.
- The symbols of the units do not take plural form. For example: 10 kg not as 10 kgs.
- When temperature is expressed in kelvin, the degree sign is omitted. For example : 273 K not as 273° K (If expressed in Celsius scale, degree sign is to be included. For example 100° C and not 100 C).
- Use of solidus is recommended only for indicating a division of one letter unit symbol by another unit symbol. Not more than one
solidus is used. For example : m s−1 or m / s, J / K mol or J K–1 mol–1 but not J / K / mol. - Some space is always to be left between the number and the symbol of the unit and also between the symbols for compound units
such as force, momentum, etc. For example, it is not correct to write 2.3m. The correct representation is 2.3 m; kg m s–2 and not as kgms-2. - Only accepted symbols should be used. For example : ampere is represented as A and not as amp. or am ; second is represented as s and not as sec.
- Numerical value of any physical quantity should be expressed in scientific notation. For an example, density of mercury is 1.36 × 104 kg m−3 and not as 13600 kg m−3.

